Exploring novel markers of embryo quality;
Elucidating the molecular mechanism of extracellular vesicles targeting
embryo maternal communication
Embryo implantation in human and animal reproduction is considered to be a very complex physiological event. The mechanisms of which is yet to be fully understood. Not only high-quality embryo but also the proper endometrial receptivity are absolutely required for proper embryo implantation. In the early phase of embryo apposition and attachment to the receptive endometrium, intense embryo-endometrial/maternal cross talks which are essential in making a favorable microenvironment for the implantation process will take place. The EVs secreted from embryo/ trophoblast cells are known to harbor specific cargo (proteins, nucleic acids, metabolites etc.) which can then be delivered to the endometrial cells to impart functional roles in the said molecular cross talks effectively. In this project, we will employ a cell culture based human embryo and receptive human endometrial surrogate model to elucidate the human embryo/trophoblast derived EVs mediation in embryo-maternal molecular cross-talks. The deep understanding of molecular mechanisms involved in the cross-talks will invariably help in finding solution for early implantation failures in both human and important farm animal species.
Project background
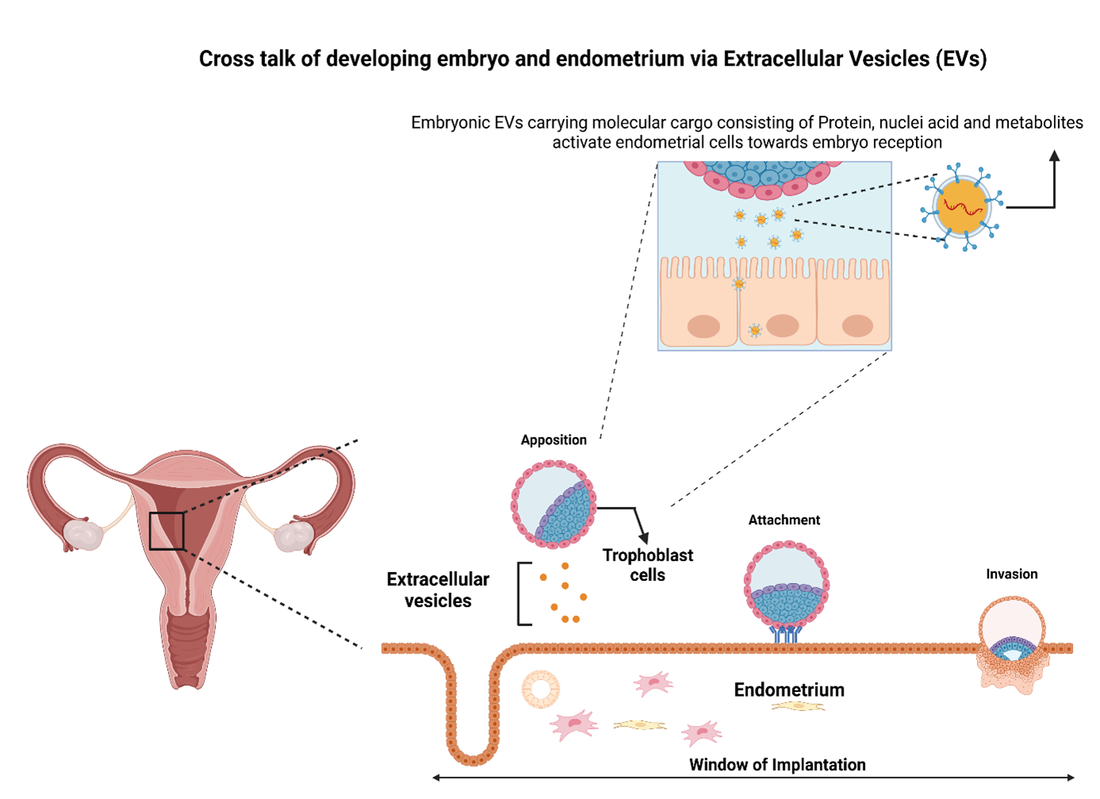
Natural embryo mortality before or during implantation is considered to be major constrain to the reproductive performance in human and other mammals. The establishment of a pregnancy is supported by reciprocatory molecular communication between the embryos and the conceiving maternal environment. EVs has been used to describe heterogeneous group of nano sized particles (30-5000 nm in size) secreted by all cell types in to the extracellular environment. These membranous vesicles were originally described as performing waste disposal from the cells, however later identified as having more important functions both in physiological and pathological status such as maintaining cellular homeostasis, cancer metastasis and intercellular signal transmission by carrying different bioactive cargoes including protein, lipids and nucleic acids to neighboring or distant recipient cells in order to reprogram it’s functions. Early two way dialogue between mother and the embryo has been shown at the level of oviduct where EVs generated from oviduct epithelial cells have shown to improve fertilization, embryo development and to prevent polyspermy. It is also noteworthy that EVs generated by different parts of the oviduct were functionally distinct and concentration of EVs also changed along different parts of the oviduct. In the uterine cavity, EVs generated by embryo exerts bio-sensing and immune modulatory effects on endometrial and immune cells supporting intercellular communication between mother and the embryo for successful implantation. EVs generated by endometrium are also internalized by trophoblasts where it modulates trophoblastic cell adhesion, invasion and migration. The biological functions exert by EVs generated from trophoblast cells on endometrial cells have been dependent on the embryo quality where the functional consequences of EV molecular cargo by good embryo was not the same as degenerating embryo. Hence there are sufficient evidence to suggest the existence of EVs mediated embryo maternal communication during early pregnancy which is also dependent on source and composition of EVs. However, the exact molecular mechanism of how EVs exert or regulate its biological functions during pre-implantation is not well studied. Es-Haghi et al. in 2019 have demonstrated that EVs deriving from JAr cells can alter the gene expression of RL95-2 cells that are commonly used in in vitro analogues of trophoblast and endometrial epithelial cells. The evidence on structural or functional consequences or specificity of altered gene expression induced by EV transfer to these cells are lacking. Hence, more studies are required to decide whether the effects of trophoblast derived EVs target endometrial cells and exert specific biological/functional effects. The proposed study will investigate functional consequences of trophoblast derived EV on endometrial cells at proteomic level to understand what biological processes are affected by EV and to investigate the involvement of different EV molecular cargo components (protein, RNA and lipid) in regulation of these biological processes. The presumed molecular communication between the trophoblast and the endometrial cells will be investigated by deep proteomic analysis of JAr EV treated RL95-2 cells that will enable identifying targets in endometrial cells for EV. The results will be used to determine the functional specificity of EV mediated embryo maternal communication during pre-implantation by comparing the effects of non-reproductive cell EVs on RL95-2 cell proteome as well. The specific signaling networks triggered by EVs in endometrial cells will be further studied with relation to EV biology to understand the mechanisms of action and molecules involved in these processes. This in-depth understanding about early embryo maternal communication via EVs will enable identification of special protein markers to detect quality of the embryo. It is assumed that good quality embryos with higher chance of implantation success will strongly activate endometrial cell cellular response to embryo derived EVs. Hence, the findings of this study will also enable optimizing assisted reproductive technologies both in humans and in other mammals and even for developing new contraceptive and therapeutic strategies (Figure 5).
Natural embryo mortality before or during implantation is considered to be major constrain to the reproductive performance in human and other mammals. The establishment of a pregnancy is supported by reciprocatory molecular communication between the embryos and the conceiving maternal environment. EVs has been used to describe heterogeneous group of nano sized particles (30-5000 nm in size) secreted by all cell types in to the extracellular environment. These membranous vesicles were originally described as performing waste disposal from the cells, however later identified as having more important functions both in physiological and pathological status such as maintaining cellular homeostasis, cancer metastasis and intercellular signal transmission by carrying different bioactive cargoes including protein, lipids and nucleic acids to neighboring or distant recipient cells in order to reprogram it’s functions. Early two way dialogue between mother and the embryo has been shown at the level of oviduct where EVs generated from oviduct epithelial cells have shown to improve fertilization, embryo development and to prevent polyspermy. It is also noteworthy that EVs generated by different parts of the oviduct were functionally distinct and concentration of EVs also changed along different parts of the oviduct. In the uterine cavity, EVs generated by embryo exerts bio-sensing and immune modulatory effects on endometrial and immune cells supporting intercellular communication between mother and the embryo for successful implantation. EVs generated by endometrium are also internalized by trophoblasts where it modulates trophoblastic cell adhesion, invasion and migration. The biological functions exert by EVs generated from trophoblast cells on endometrial cells have been dependent on the embryo quality where the functional consequences of EV molecular cargo by good embryo was not the same as degenerating embryo. Hence there are sufficient evidence to suggest the existence of EVs mediated embryo maternal communication during early pregnancy which is also dependent on source and composition of EVs. However, the exact molecular mechanism of how EVs exert or regulate its biological functions during pre-implantation is not well studied. Es-Haghi et al. in 2019 have demonstrated that EVs deriving from JAr cells can alter the gene expression of RL95-2 cells that are commonly used in in vitro analogues of trophoblast and endometrial epithelial cells. The evidence on structural or functional consequences or specificity of altered gene expression induced by EV transfer to these cells are lacking. Hence, more studies are required to decide whether the effects of trophoblast derived EVs target endometrial cells and exert specific biological/functional effects. The proposed study will investigate functional consequences of trophoblast derived EV on endometrial cells at proteomic level to understand what biological processes are affected by EV and to investigate the involvement of different EV molecular cargo components (protein, RNA and lipid) in regulation of these biological processes. The presumed molecular communication between the trophoblast and the endometrial cells will be investigated by deep proteomic analysis of JAr EV treated RL95-2 cells that will enable identifying targets in endometrial cells for EV. The results will be used to determine the functional specificity of EV mediated embryo maternal communication during pre-implantation by comparing the effects of non-reproductive cell EVs on RL95-2 cell proteome as well. The specific signaling networks triggered by EVs in endometrial cells will be further studied with relation to EV biology to understand the mechanisms of action and molecules involved in these processes. This in-depth understanding about early embryo maternal communication via EVs will enable identification of special protein markers to detect quality of the embryo. It is assumed that good quality embryos with higher chance of implantation success will strongly activate endometrial cell cellular response to embryo derived EVs. Hence, the findings of this study will also enable optimizing assisted reproductive technologies both in humans and in other mammals and even for developing new contraceptive and therapeutic strategies (Figure 5).
Figure 5. A schematic presentation of different aspects of cross talk between the embryo and mother utilizing extracellular Vesicles.
Project objectives
- To analyze the endometrial epithelial cell proteomic response to trophoblast cell derived EVs using in vitro cell culture model consisting of RL95-2 (analogue of endometrial epithelial cells) and JAr cells (analogue of trophoblast cells)
- To identify the Protein Cargo of trophoblast cell derived EVs (JAr cells) compared to non-trophoblastic cell derived EVs and their role in embryo maternal communication during pre-implantation
- To study the specific molecular signaling pathways involved in EV mediated embryo maternal communication during pre – implantation using multiple molecular techniques
Integration with EMU expertise, research strategy and development
EMU has a strong research program in relation to reproductive physiology and biology. This is related to the long roots of reproductive research in EMU. The history of the Department of Reproductive Biology dates back to 1956 when the Laboratory of Artificial Insemination was founded as one of the seven subunits established at the Estonian Research Institute of Animal Breeding and Veterinary Science by a decree of the Ministry of Agriculture of the Estonian SSR. After the merger of the Estonian Research Institute of Animal Breeding and Veterinary Science and the Estonian Agricultural University in 1994, the Department was incorporated into the Faculty of Veterinary Medicine of the University. On 1 September 2017, the Department of Animal Genetics and Breeding and the Department of Reproductive Biology were merged to establish the Chair of Animal Breeding and Biotechnology in EMU.
The Chair of Animal Breeding and Biotechnology is Estonia’s only competence centre in its field. It offers learning opportunities at all levels of higher education. Basic and applied research involving breeding of livestock, preservation of animal genetic resources, animal genetics, genomics, transgenic technology, reproduction and reproductive disorders, and reproduction biotechnology.
Understanding the mechanisms behind causes of infertility in human as well as infertility and subfertility in animals is a very important aspect of comparative medicine and one health approach. The current research project is well in line with aims of the Combivet project to support research in human and animal based common diseases as well as understanding the mechanism of disease to help Human and animals. All these points are very important in upgrading the quality of research currently performed in EMU.
EMU has a strong research program in relation to reproductive physiology and biology. This is related to the long roots of reproductive research in EMU. The history of the Department of Reproductive Biology dates back to 1956 when the Laboratory of Artificial Insemination was founded as one of the seven subunits established at the Estonian Research Institute of Animal Breeding and Veterinary Science by a decree of the Ministry of Agriculture of the Estonian SSR. After the merger of the Estonian Research Institute of Animal Breeding and Veterinary Science and the Estonian Agricultural University in 1994, the Department was incorporated into the Faculty of Veterinary Medicine of the University. On 1 September 2017, the Department of Animal Genetics and Breeding and the Department of Reproductive Biology were merged to establish the Chair of Animal Breeding and Biotechnology in EMU.
The Chair of Animal Breeding and Biotechnology is Estonia’s only competence centre in its field. It offers learning opportunities at all levels of higher education. Basic and applied research involving breeding of livestock, preservation of animal genetic resources, animal genetics, genomics, transgenic technology, reproduction and reproductive disorders, and reproduction biotechnology.
Understanding the mechanisms behind causes of infertility in human as well as infertility and subfertility in animals is a very important aspect of comparative medicine and one health approach. The current research project is well in line with aims of the Combivet project to support research in human and animal based common diseases as well as understanding the mechanism of disease to help Human and animals. All these points are very important in upgrading the quality of research currently performed in EMU.
Project team
|
Subhashini Muhandiram
Institute of Veterinary Medicine and Animal Sciences Student |
Alireza Fazeli
Institute of Veterinary Medicine and Animal Sciences Supervisor |
|
Suranga Kodithuwakku
Institute of Veterinary Medicine and Animal Sciences Supervisor |
Kasun Godakumara
Institute of Veterinary Medicine and Animal Sciences Supervisor |